Clinical Studies
Detection of HIV proviral DNA on Toothbrushes: a preliminary study.
- Richard T. Glass, DDS, PhD
- Steven R. Carson, DDS, MEd
- Robert L. Barker, PhD
- Stephen C. Peiper, MD
- Stewart Shapiro, DMD,MScH,PhD
In 1986, Glass and Lare published the results of a systematic study concerning microorganisms found on the toothbrushes of "healthy" dental patients; patients with inflammatory disease; and as controls, new toothbrushes taken directly from their packages1.
The investigation reported that:
(1) microorganisms found on toothbrushes from the inflammatory disease patients were those known to produce
either local (dental) or systemic diseases;
(2) pathogenic microorganisms were found on the toothbrushes of the "healthy" dental patients; and
(3) four out of five (80%) of the toothbrushes from one manufacturer were contaminated in the package.
These findings were confirmed by Kozai et al who examined toothbrushes from 150 children and found large quantities of microorganisms were present even thorough rinsing and 24 hours of drying2. Subsequent investigation by Glass and Jensen were performed to delineate the problems of the toothbrushes further or to identify methods and/or technique3. They inoculated known quantities of Herpes Simplex Virus, Type 1 (HSV-1) onto sterile numbers of infections viruses (as determined by cytopathic effect on tissue culture cells) could be retrieved after 48 hours from artificially dried toothbrushes (HSV-1 is usually killed by drying). When toothbrushes were maintained in a moist environment (similar to that of a bathroom), approximately 50% of the original number of virus could be retrieved after seven days. Vital staining of the microorganisms revealed the majority of viruses adhered to defects in the bristles (both shaft and ends) and the cores of natural bristles toothbrushes. These positions of adhered often were associated wit proximate jagged bristles edges. Thus, two of the necessary criteria for transmission of disease were met: (1) presence of viable microorganisms and (2) a potential portal of entry.
Disease
transmission from contaminated toothbrushes was demonstrated in an
animal model study4. Using dogs, repeated brushes with
the same toothbrushes produced more dental disease than either
brushing with a sterile toothbrush or brushes with a toothbrush
contaminated with known pathogens. The importance of that study was
that it confirmed (1) even sterile toothbrushes produce musosal
abrasions and subsequent disease and (2) toothbrushing introduces
microorganism into vessels. While a number of epidemiologic
studies have pointed out that non-percutaneous, non-sexual modes of
HIV transmission are rare, four case reported have described such
transmissions5,6,7,8,9,10,11,12. The most recent reports
noted that children who share toothbrushes had gingival hemorrhage
on brushing13. The lay media (U.S. News & World
Report) has alerted the public and "placed the public on guard" as
to the potential transmission of HIV in the home setting, implicates
the toothbrush as a possible vector14. Possible HIV
transmission by dental means concentration has been confounded by
reports of high titters of HIV antibodies in mucosal transudates
contrasted with studies that have demonstrated an HIV blocking or
neutralizing effect by saliva15,16,17,18,19,20,21. Even
though the toothbrush comes into contact with saliva, it has also
been found to retain both mucosal and inflammatory cells. The
toothbrush, therefore, may harbor HIV and needs to be examined as a
possible vector in HIV transmission. The following study was
conducted to answer the question:
Can HIV proviral DNA
be isolated from the toothbrushes of known HIV positive
patients?
Materials
and Methods
This
exploitative study was designed to be a single blind study. It was
projected to include at least six HIV positive subjects as study
cases and two HIV negative subjects as controls. The toothbrushes
used daily by the subjects were obtained for further analysis for
contaminated with HIV proviral DNA. In addition, a second control
group was comprised of two toothbrushes that were never used by a
patient.
Therefore, then study was comprised of ten toothbrushes:
six from HIV positive patients, two from non-infected individuals,
and two new toothbrushes. All patients were asked to bring their
toothbrush to a dental examination. Informed consent was obtained, a
screening examination performed, and significant data (i.e.
toothbrush environment, oral health status, etc.) collected. A
control patient was considered "clinically healthy" if, following
clinical examination by the principal investigator, they were found
to be caries free and were not found to have mucosa disease not
gingival/periodontal inflammatory disease.
When the patients
arrived with their toothbrushes, sterile technique was used to
remove a bundle of bristles. The bristle were then placed in a
sterile glass tube with a lid and stored at -60?. Following
collections of all samples, the tubes were packed in dry ice and
shipped to the University of Louisville where they were tested using
three different polymerase chain reaction (PCR) techniques for
presence of HIV proviral DNA22.
The sequence of the
HIV PCR primers (JA17-JA20) were followed according to the
techniques reported by Albert and Fenyo23. The primers
were synthesized using an Applied Biosystems 391 EP synthesizer and
purified over Sephadex G-25 columns. A nested PCR was utilized (In
nested PCR, the target DNA was amplified with a specific primer set.
Ten percent of this first reaction was used as template for a second
PCR containing a set of specific primers JA 17 and JA 20. Ten
percent of that reaction was then used template in a PCR using
primers JA18 and JA19. The final reactions were then resolved on a
2% agarose TAE gel containing ethidium bromide that binds DNA and
can be visualized under UV illumination. An amplification reaction
was considered positive if a 111 basepair DNA amplification products
were detected.
A Perkin-Elmer Cetus 9600 GeneAmp PCR system
thermal cycle was used to incubate the samples. Both reactions of a
nested PCR were amplified for 30 cycles each. Each cycle for both
reactions consisted of a 30 second denaturation step at 94?,
followed by a 30 second annealing step at 41?, and finally a 30
second extension step at 72?.
In each PCR test group, DNA
from an HIV positive individual was used as a positive control,
while placental DNA was used as a negative control. In the first
round of tests, 10 ul from each template treatment (TE and K buffer)
from each sample was used as a separate template in a nested HIV
PCR.
In the next set of tests, each K buffer treated sample was
concentrated by vacuum centrifugation and the entire sample was used
in PCR. Although this increased the amount of DNA added to the
reaction , it also increased the salt concentration.
One-third of
the bristles from each sample were incubated with 200 microliters of
TE (10mM Tris-HCI, 1mM EDTA, pH 8.0) at 100? for ten minutes.
Another one-third of the bristles were incubated with 200
microliters of K buffer (50mM KCI, 10mM Tris-HCI, 2.5mM Magnessium
Chloride, pH 8.3 with 0.5% Tween 20 and 100ug/ml Proteinase K) for
one hour at 56? followed by ten minute 100? incubation to inactive
the protease. The remaining bristle were stored at -20?.
Finally,
the remaining untreated bristles were cut into small pieces and
treated with 50ul of K buffer. 10ul of each sample of this
preparation was used for PCR
amplification.
Results
Single blind study of toothbrushes using PCR techniques was
performed on HIV positive patients, normal subjects and new
toothbrushes. The HIV positive subjects were male with an age range
of 31-39 years of age (average age= 33.6 years). The HIV negative
toothbrushing controls consisted of a male (age, 35 years) and one
female (age, 42 years). Four of the HIV positive patients
demonstrated mucosal inflammatory disease; one of HIV positive
patients had periodontal disease; and two of the HIV positive
patients had caries. The control met the criteria of not having any
active dental diseases.
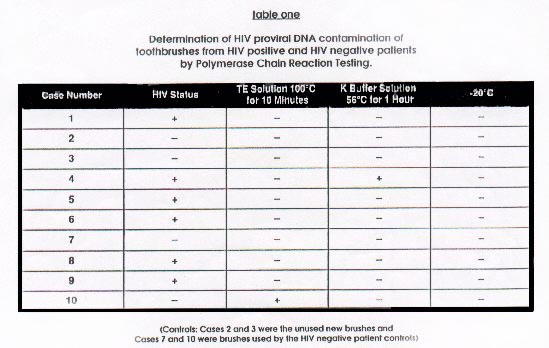
The results of the PCR analysis of
toothbrushes using three separate techniques are summarized in Table
1. Using the TE solution at 100? for ten min. method, only case #10
(a toothbrush from a "clinically healthy", HIV negative subject) was
found positive for HIV proviral DNA. Using the K buffered solution,
case #10 was negative, but case #4 (a toothbrush from an HIV
positive subject with active periodontal disease) was positive for
HIV proviral DNA. None of the bristles which had been stored (dried)
at -20? were PCR positive for HIV proviral
DNA.
Discussion
Although the intent of this study addressed the question of
transmission of HIV proviral DNA via the toothbrush, the issue of
detection of HIV proviral DNA on the toothbrush and the use of PCR
testing was fundamental to this point exploratory project. PCR
testing is a extremely sensitive procedure and can be affected by
the slightest contaminated, which can impact the potential value of
PCR testing as a screening tool. Although the results of this
project are based upon a small sample size, the concerned of false
positive and/or negative arise.
There were three phases or tests
in the PCR programming, namely the TE solution, the K buffer
solution, and the 20? or drying phase. The TE phases demonstrated 6
false negative and 1 false positive; the K buffer solution also
demonstrated 5 false negatives, but did not identify any false
positives; and the -20? demonstrated 6 false negatives and no false
positives. It would appear from the results of the limited sample in
this study that the high level of false negatives would adversely
the interpretation of the role of the toothbrush in the transmission
of HIV proviral DNA. The issue then emerges as to whether PCR
testing is sensitive enough or too sensitive to identify HIV
proviral DNA on the toothbrush. While one toothbrush from an HIV
positive patient patients was found positive for HIV proviral DNA,
five toothbrushes from HIV positive patients were found negative for
HIV proviral DNA. Several possible explanations for this finding
seem reasonable: (1) HIV proviral DNA was not shed by the HIV
positive patient at the time of the study; (2) HIV proviral DNA was
being destroyed by the saliva; or (3) HIV proviral DNA was being
destroyed consistently retained on the toothbrushes.
Based upon
pervious investigation by the author, the contamination and disease
transmission by the toothbrush have been clearly demonstrated.
Therefore, the issue highlighted by this study emphasizes the need
for education concerned toothbrush care for AIDS patients and their
families. This need has been brought to the forefront by the recent
high media coverage regarding transmission of the virus to family
members through an unknown vector.13,14. As pointed out
in the Editorials of the same issue of the New England Journal of
Medicine [Ref.13], an important component in a protocol for the home
environment of an HIV positive individual must be elimination and
control of all potential vector of HIV proviral DNA transmission
24. Since toothbrush contamination and disease
transmission has already been demonstrated, good toothbrush care
must be a part of any preventive program1,3,4. Good
toothbrush care would include a new toothbrush every two weeks;
storage of the toothbrush in a dry environment outside the bathroom;
and the most obvious, not sharing toothbrushes
25,26.
The toothbrush method of HIV proviral DNA
detection needs further investigation. This will allow
determinations to whether the HIV proviral DNA from the toothbrush
can be infectious. Also, in a constant search for techniques for the
early detection of HIV, the contaminated toothbrush may play an
important role if it can be found that the toothbrush is positive
during the "window period" of HIV
infection.
Conclusion
PCR testing for HIV proviral DNA on the toothbrush is in the
developmental stage. This may explain the inconsistencies of the
results obtained in this study. Further investigation is needed to
refine the procedures used to detect the presence of HIV proviral
DNA from the toothbrushes from HIV positive patients is needed to
evaluate the sensitivity of PCR testing relative to limiting the
percentage of false negatives. Based upon previous data, the
potential for transmission for the HIV proviral DNA via toothbrush
is a viable consideration in controlling infection. The role of PCR
testing as a screening procedure may not yield satisfactory
sensitivity or specificity levels, but this does not, and should
not, rule out the potential for transmission of HIV proviral DNA via
this substantiated disease transmission vector. As part and parcel
of any home regimen associated with the control of reinfection or
transmission of HIV, appropriate attention, must be directed at
maintaining good toothbrush hygiene.
Featured Articles
Read MoreCustomer Testimonials
-
- "Purebrush provides the protection they need..."
- "Because people forget to change their toothbrushes as often as they should, Purebrush provides the protection they need."
-
- "Dr visits have decreased dramatically..."
- Purebrush is wonderful! I have two small children and our doctor visits have decreased dramatically since we purchased our Purebrush. I really think your product is great and I believe that you are doing a great thing for people's health.
Read More




